Addressing The Impacts of PFAS in Biosolids
By Johnathan Sheets and Maddison Ledoux | Water & Wastes Digest | September 10, 2021
Read the full article by Johnathan Sheets and Maddison Ledoux (Water & Wastes Digest)
“In recent years, per- and polyfluoroalkyl substances (PFAS) have become a topic of public concern, particularly when discovered in drinking water supplies. PFAS are a family of more than 3,000 man-made chemicals that have been manufactured and used since the 1940s. This large class of fluorosurfactants have unique chemical and physical properties, which make them extremely persistent, as well as mobile, in the environment.
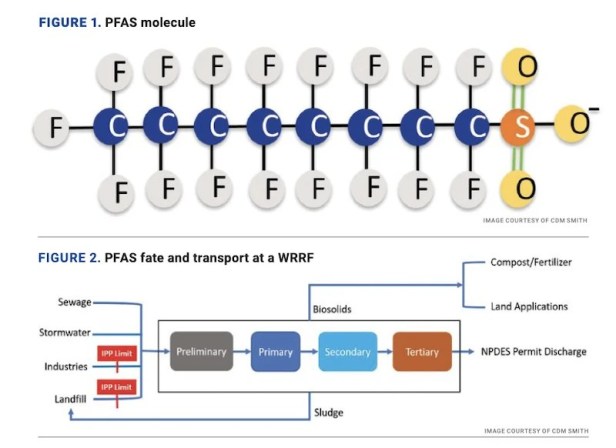
The carbon-fluorine (C-F) bond in PFAS is the strongest bond in chemistry which does not break down naturally in the environment. Long-chain PFAS, specifically perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), have been phased out since 2002. Many replacement compounds are poly-fluorinated and could degrade into precursors of long-chain legacy compounds. The prevalence of PFAS in the environment has raised concerns about the possibility of their adverse health impacts. An illustrative example of a PFAS molecule is shown in Figure 1.
PFAS in Biosolids
PFAS compounds are not directly generated at water resource recovery facilities (WRRFs), but rather enter WRRFs from other sources. Examples include through wastewater generated at industrial facilities that produce or process PFAS, through leachate from landfills that contain PFAS-laden wastes, through municipal wastewater with background levels of PFAS and through contaminated storm water, among others. According to the Interstate Technology Regulatory Council (ITRC), typical treatment methods at WRRFs do not remove or destroy PFAS, and a portion of those compounds may partition to sludge. Figure 2 describes the PFAS fate and transport at a typical WRRF.
Common sludge treatment processes, such as lime treatment, digestion, thermal drying, and composting do not reduce PFAS in sludge. Therefore, PFAS are present in both plant effluent and in biosolids at U.S. WRRFs. The most common PFAS compounds in sludge are PFOS (<10 to 1,100 ng/g dry weight) and PFOA (1 to 240 ng/g dry weight). As expected, the concentration of PFAS in biosolids is higher at WRRFs that serve industrial customers.
In 2004, a North East Biosolids and Residuals Association (NEBRA) survey showed that approximately 55% of wastewater solids are recycled to soils as biosolids, about 30% are landfilled, and about 15% are incinerated. Each of these management options may lead to environmental releases of PFAS. For example, PFAS may transport from landfilled solids to groundwater (unlined landfills) or leachate (lined landfills). Low temperature incineration may cause PFAS to be transferred from the solid matrix to the air. PFAS may also be released from land applied biosolids to the soil matrix, groundwater or surface runoff.
However, the fate and transport of PFAS from biosolids is dependent on several factors. The type and concentration of PFAS must be well understood since the compounds are known to have varying physical and chemical properties. Site characteristics, such as soil properties, weather patterns, plant uptake, and biosolids application methods, impact environmental transport of PFAS. Agencies such as the U.S. EPA Office of Research and Development, the Water Research Foundation (WRF), and several others are investing in research to improve knowledge of PFAS transport and provide guidance to regulators.”…
This content provided by the PFAS Project.
Location:
Topics: